Yena Lee
Learn how to launch promotional banners and sales content seamlessly across your site without developer bottlenecks. We cover how to audit your sales content workflows to create a headless e-commerce roadmap that works for your brand and walk through examples of how we’ve solved sales campaigns for major e-commerce brands.

A practical guide to using AI to curate product collections at scale, make product recommendation and search algorithms smarter with semantic awareness, and empower teams to deliver seamless customer experiences.

Generate nuanced translations for your e-commerce site using AI. Learn how to set up, fine-tune, and integrate AI models seamlessly with your headless CMS for automatic content translation. Uncover practical steps for implementation, from choosing the right language model to architecting efficient translation workflows that align with your budget and goals.
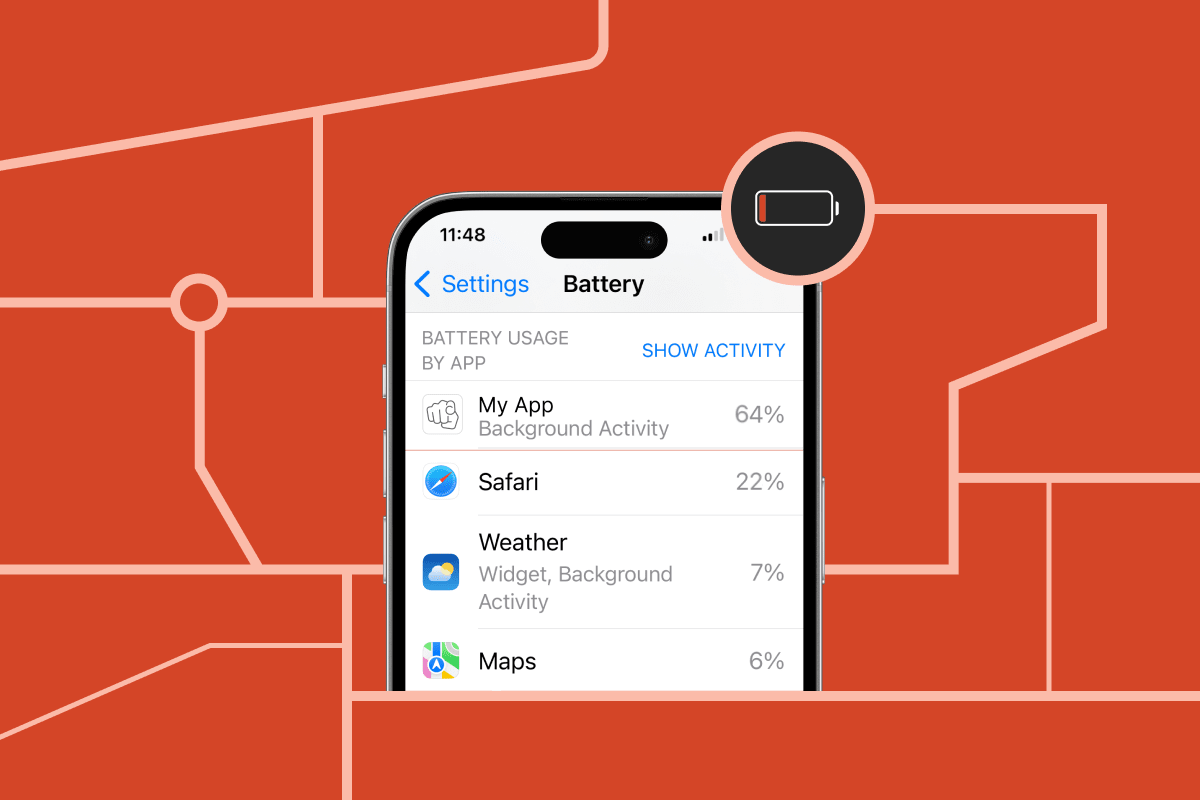
Lowering location accuracy is not the best way to reduce your app's power usage. Use these configurations for iOS background location services and stop draining your users' batteries.
Choosing a headless CMS can be a daunting task. We created a tool for you to chat with our headless platform expert Scott Fuller to help you find the best headless solution for your business.
Contentful, Sanity, and Strapi are the headless CMS platforms we recommend most often to our clients. Here's a comparison of features that matter, plus an in-depth guide to choosing the right headless CMS platform for you.

A headless CMS offers more flexibility to integrate with a variety of front-end solutions and devices, making it an excellent option for highly scalable and performance-focused websites. Non-technical team members can edit content, optimize for SEO and performance, and use the same content across different channels and touch points.

Plus, what AI won’t tell you about how to choose a headless CMS. Read how Sanity, Contentful, and Strapi compare on features that matter.







