
Healthcare
Use Digital to Disrupt Healthcare
We work closely with telemedicine and software as a medical device scale-ups, as well as market leading pharmaceutical companies. Our experts in health tech markets create compliant digital products with superb experiences that deliver value for patients and HCPs in weeks, not years.
How we can help
We've helped Health Tech & Pharma firms of all sizes truly innovate and transform in ways that get measurable results.
Disruptive Innovation
Software as Medical Device
Compliance by Design
When it comes to healthcare innovation, it's no longer only about digitalizing processes and improving experiences.
There are exponential advances in mobile and wearable technology providing brand new digital opportunities to utilise untapped behavioural data, influence how users consume digital products and build streamlined digital ecosystems.
The early winner of the digital healthcare transformation wave was definitely the telemedicine industry, but SaMD is going to be bigger.
While telemedicine keeps on growing with a predicted compound annual growth rate of 16% between 2020-2027, Software as Medical Device (SaMD) represents an even bigger opportunity – an estimated 22% growth – for creating profitable digital products with a purpose.
Organizations can integrate GRC processes as part of an end-to-end, trans-disciplinary process and launch regulated digital health products on average 60% faster.
Regulatory compliance is much more of a process challenge than a tooling issue. Long lead times are often the result of a siloed approach to risk and compliance and our Compliance by Design approach solves for this.
Disruptive Innovation
When it comes to healthcare innovation, it's no longer only about digitalizing processes and improving experiences.
There are exponential advances in mobile and wearable technology providing brand new digital opportunities to utilise untapped behavioural data, influence how users consume digital products and build streamlined digital ecosystems.
Software as Medical Device
The early winner of the digital healthcare transformation wave was definitely the telemedicine industry, but SaMD is going to be bigger.
While telemedicine keeps on growing with a predicted compound annual growth rate of 16% between 2020-2027, Software as Medical Device (SaMD) represents an even bigger opportunity – an estimated 22% growth – for creating profitable digital products with a purpose.
Compliance by Design
Organizations can integrate GRC processes as part of an end-to-end, trans-disciplinary process and launch regulated digital health products on average 60% faster.
Regulatory compliance is much more of a process challenge than a tooling issue. Long lead times are often the result of a siloed approach to risk and compliance and our Compliance by Design approach solves for this.
Companies we've helped
Software as a Medical Device

Roche
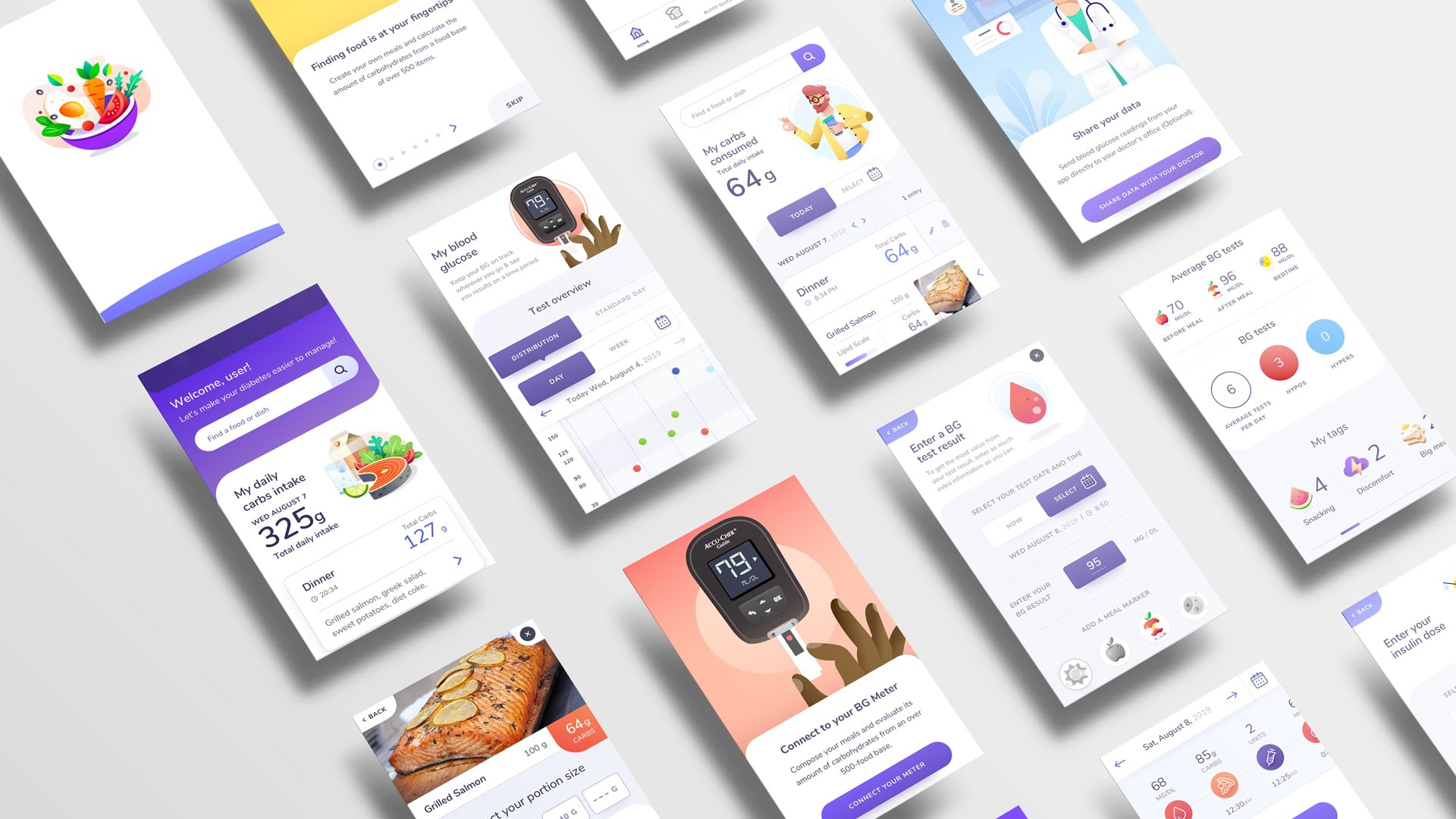
9 months to launch the world's first Javascript React Native app with CE marking as a class II SaMD
The client needed to create a new digital product to help people with diabetes.
Headless CMS

hims & hers
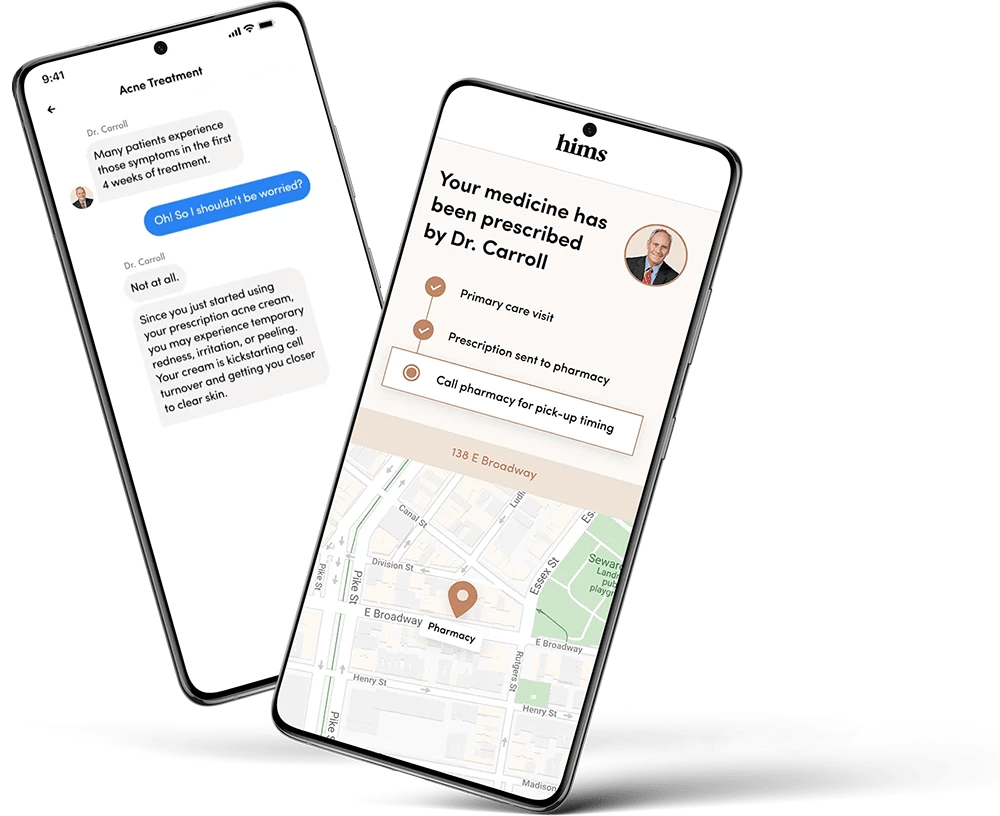
4 weeks to market with a new headless e-commerce platform
Hims needed a scalable telehealth platform to become a one-stop shop for medical advice and treatment. We helped them go headless and launch Hers into new markets in 30-days.
New Product Development & Artificial Intelligence

Babbly
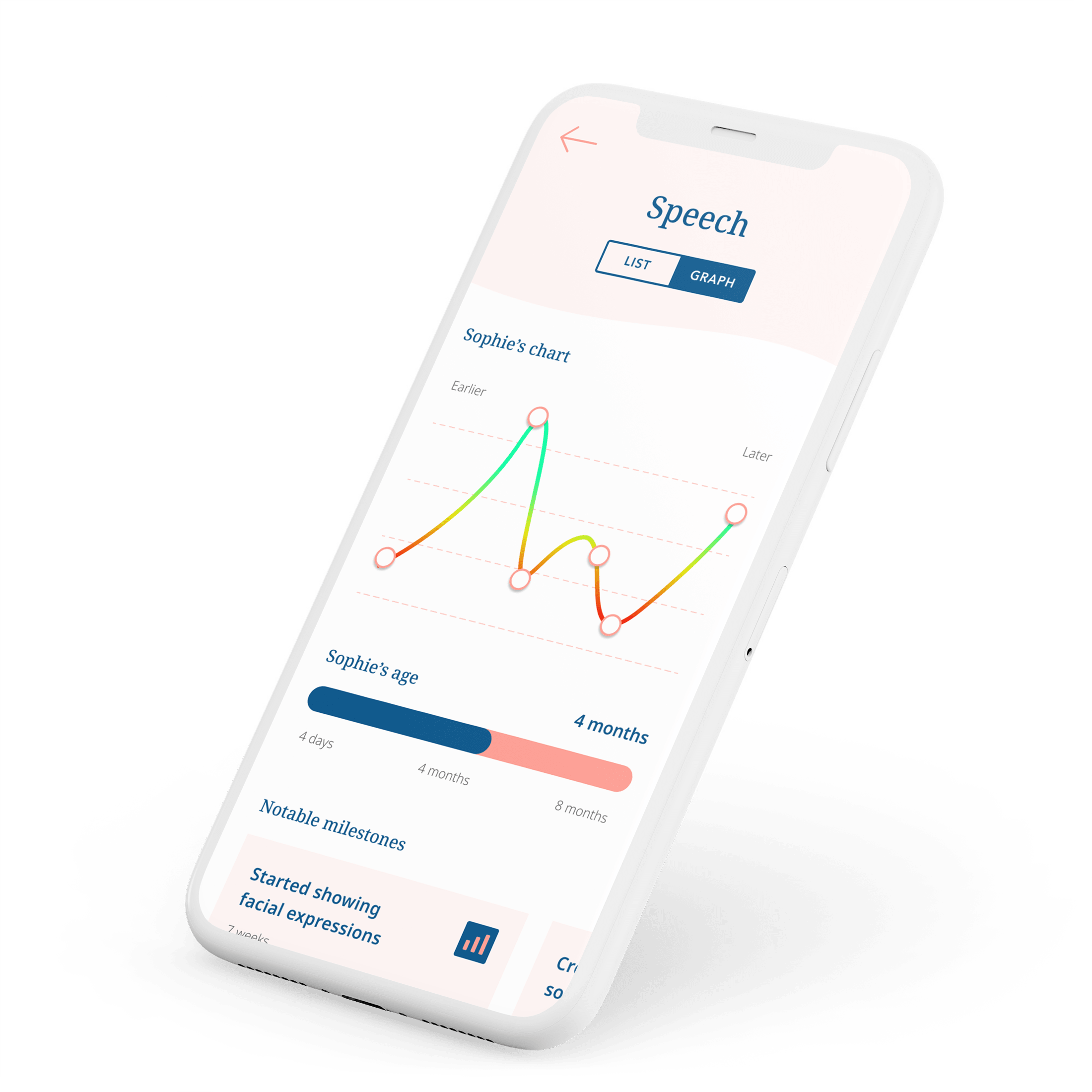
An AI-enabled demo from concept to functional model in just 2 weeks
Babbly team closed a successful pre-seed investment round with machine learning PoC.






