
9 months to launch the world's first Javascript React Native app with CE marking as a class II SaMD
Highlights
- Idea to POC in 4 weeks: created an innovative patient-centric app for Roche
- Successfully applied Compliance by Design to healthcare, including regulatory, quality, medical affairs and legal processes; completed the client’s two-year process in 9 months
- First Javascript React Native app in the world to achieve CE marking as a class II SaMD
The challenge
Our client set a goal to create a new digital product to help people with diabetes to better manage their condition and support them in achieving goals they set with their healthcare professionals. The product had to be capable of evolving to meet the changing needs of customers, while meeting regulatory requirements for software as a medical device (SaMD). In order to be marketed as a medical device in Europe, the client needed to demonstrate that their digital product met all EU regulatory requirements for CE marking.
The process
We partnered with the client's team to help patients with diabetes better manage their condition. As we were building a product with medical purposes for patients, we needed to adhere to requirements and standards for designing and developing SaMD (e.g., ISO 13485, IEC/ISO 62304, EU MDR 2017/745). In addition, we needed to demonstrate that the app met all requirements in an audit performed by a notified body to obtain CE marking and launch the product in Europe.
We applied Compliance by Design to build software as a medical device and achieve CE marking in nine months, without a single deviation or request for change from the audit process.
A central tenet of Compliance by Design is that interactions between individuals and cross-functional teams must be prioritized over processes and tools. We created cohesion and collaboration between members from design, engineering, enterprise architecture, product, medical affairs, legal, regulatory & compliance, quality, marketing and business teams. We applied Agile to leverage the tacit knowledge and expertise of 12 stakeholders and as many as 90 team members spanning three countries and four vendors. From day one, we allowed for continuous collaboration while rapidly producing working software and managing change.
The client had previously taken two years, on average, to progress from development to certification for similarly sized digital initiatives. We helped the client teams to adopt Agile and embrace change. Agile practices such as continuous delivery, automated testing, regular customer verification and validation, and design reviews, were critical to aligning teams to adopt an evolutionary software life cycle.
Compliance by Design
At the outset of building the initial prototype, we engineered the software system architecture to compartmentalize the app–a Class IIa medical device–into software items with separate safety classifications.
Segregating the software system allowed our teams to isolate components, so that changes relating to the look and feel of the app, for example, would not trigger an audit of the entire app or be scrutinized as changes to a Class IIa medical device.
By insulating disparate features, we also lessened the burden of documentation and review throughout the development process; our teams could build features more quickly. This way of engineering the app’s architecture also enabled our teams to incrementally and iteratively build features as our understanding of user needs evolved.
The combination of working with the regulatory compliance team from day one and our transparent software system architecture enabled us to flag risks as part of the Corrective and Preventive Actions (CAPA) process and closely align on other standard operating procedures (SOPs) and quality management system (QMS) processes. This enabled us to build trust and confidence-to-ship with stakeholders, as well as the notified body.
Read more about Compliance by Design.
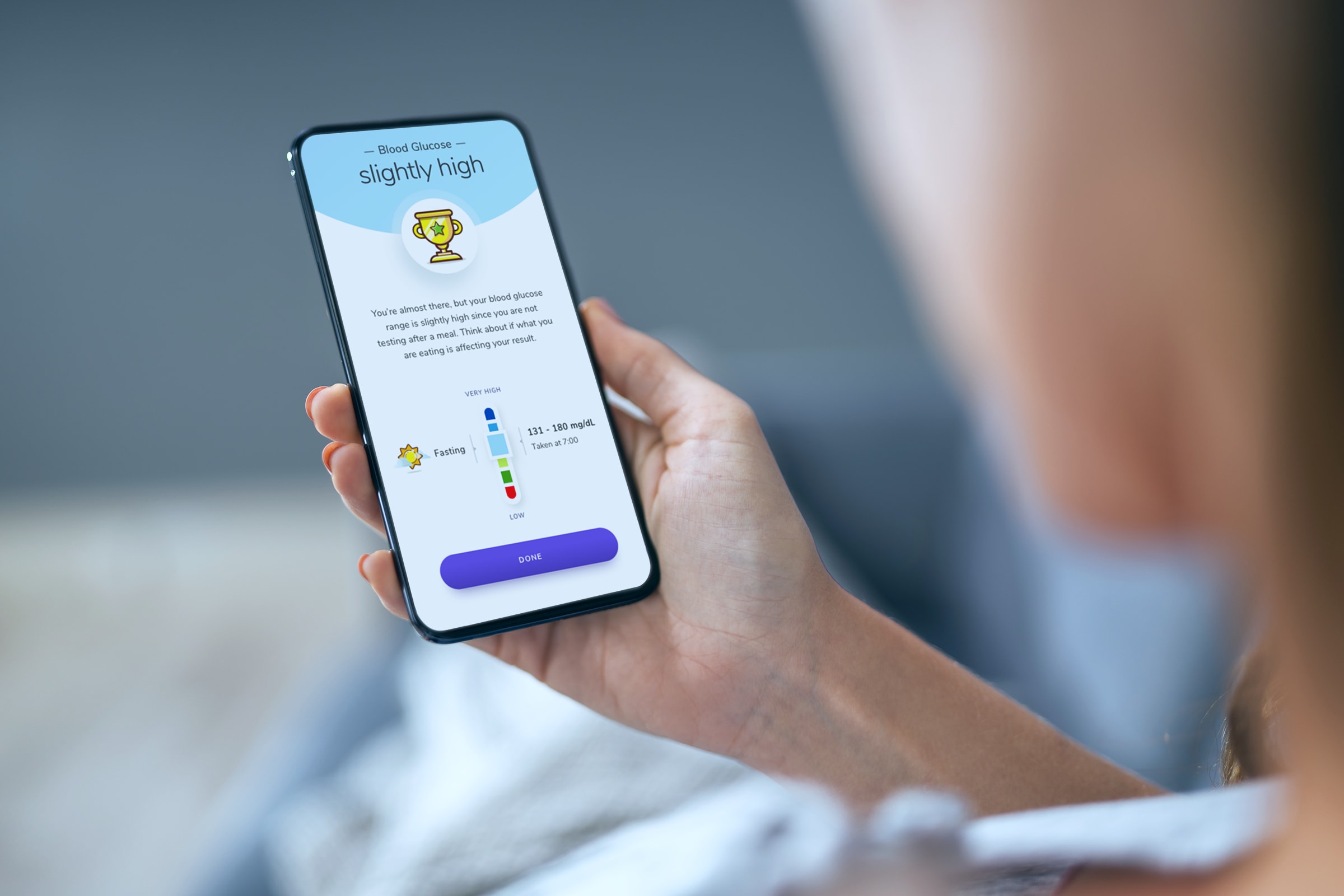
The outcome
In partnership with the client, we created the world’s first software as a medical device that determines blood glucose readings using a phone camera without the need of a blood glucose meter. The product helps non-insulin dependent people with type 2 diabetes or pre-diabetes live healthier lives through learning how their everyday actions change their blood glucose range and supporting patients to achieve the goals they set together with their healthcare professional.
A user-friendly, step-by-step guide takes users through the testing process. Next, a dedicated algorithm determines the patient’s blood glucose reading and prompts the patient to take action, based on the target health outcomes (e.g., blood glucose range) set by them and their physician. The app uses big data and provides patients with evidence-based recommendations and insights about dietary and lifestyle choices that are impacting their healthcare conditions.
The digital product is now an in vitro diagnostic medical device for mobile platforms, and provides patients globally with access to affordable and convenient diabetes management without the need for specialized blood glucose measurement hardware.
At the end of 2021, the client added new features that would trigger another review and audit of the entire app, not just the recent changes. By applying Compliance by Design, we delivered the new changes faster and without any deviation or change request from the audit process.